Clinical trials for first-line therapies in NSCLC
Approved immunotherapeutic strategies for treatment-naive patients now include monotherapy, immunotherapy-exclusive regimens or chemotherapy–immunotherapy combinations. Decision making in this space is complex given the absence of head-to-head prospective comparisons, although a thorough analysis of long-term efficacy and safety data from pivotal clinical trials can provide insight into the optimal management of each subset of patients.
First-Line Metastatic Disease
Pembrolizumab
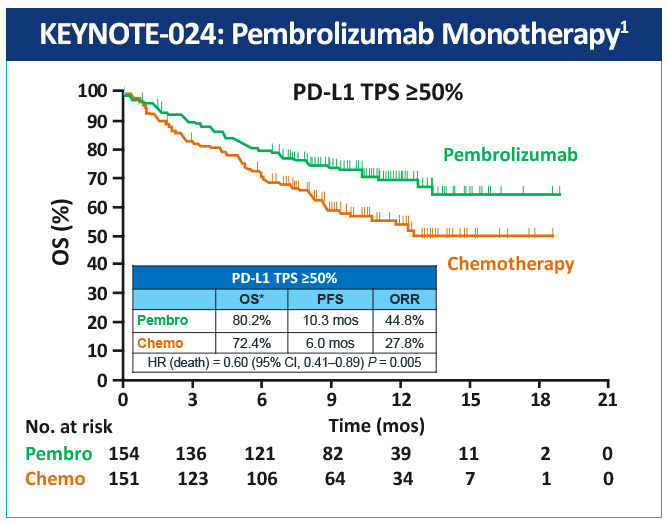
The KEYNOTE-024 study, a randomized, phase 3 study evaluated pembrolizumab as monotherapy compared with standard of care platinum-based chemotherapy in the treatment of patients with metastatic NSCLC whose tumors were marker-negative (EGFR or ALK) and expressed high levels of PD-L1 (≥50% of tumor cells).1 Median PFS was 10.3 months with pembrolizumab versus 6 months with chemotherapy; OS at 6 months was 80% vs 72% (HR=0.60); and 1-year OS was 70% vs 54%, respectively (Figure 1). Toxicity was lower with pembrolizumab than with chemotherapy (grade 3/4 adverse events: 27% vs 53%, respectively), and the incidence of all adverse events was lower with pembrolizumab. Based on the results of this study, the FDA and EMA have approved pembrolizumab for first-line treatment of patients with metastatic NSCLC whose tumors have high PD-L1 expression (TPS >50%) with no EGFR or ALK genomic tumor aberrations.
The results of KEYNOTE-021, a randomized, open-label, phase 2 study demonstrated that combination pembrolizumab, carboplatin, and pemetrexed was effective and tolerable in the first-line setting for patients with advanced non-squamous NSCLC in patients who do not express PD-L1.2 The addition of pembrolizumab to chemotherapy significantly improved PFS (19.0 vs 8.9 months) compared with chemotherapy alone. In a cohort of patients with chemotherapy-naïve, stage III or IV, non-squamous NSCLC, the addition of pembrolizumab to carboplatin and pemetrexed significantly improved ORR over chemotherapy alone (55% vs 29%; P= .0016). The most severe AEs with this combination were immune-mediated adverse events (hypothyroidism, hyperthyroidism, pneumonitis) that occur at a similar rate to pembrolizumab monotherapy.
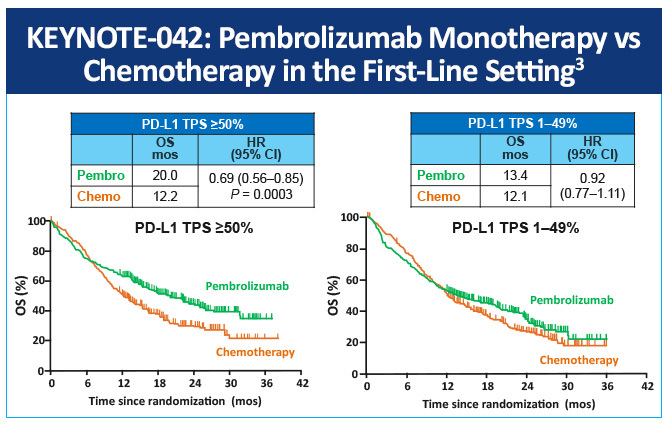
In KEYNOTE-042, a randomized, open-label, phase 3 study, first-line pembrolizumab monotherapy in patients with locally advanced or metastatic NSCLC and low PD-L1 TPS was evaluated compared with platinum-based chemotherapy of choice.3 Overall survival was significantly longer in the pembrolizumab arm than in the chemotherapy group in all three TPS populations (≥50% hazard ratio 0.69, P= .0003; ≥20% 0.77, P= .0020, and ≥1% 0.81, P= .0018) (Figure 2). The median survival values by TPS population were 20.0 months for pembrolizumab versus 12.2 months for chemotherapy, 17.7 months versus 13.0 months, and 16.7 months versus 12.1 months, respectively.
Nivolumab
The phase III, open-label, randomized study (CheckMate-057) compared nivolumab with docetaxel in advanced non-squamous NSCLC.4 This study showed an improved OS (12.2 months vs 9.4 months) and response rate (19% vs 12%) for nivolumab versus docetaxel. A subset of patients with high levels of expression of PD-L1 showed improved benefit with median OS of 17.2 versus 19.4 months. However, in patients with PD-L1-negative non-squamous cell NSCLC, the objective response rate correlated with PD-L1 expression as did the survival. The PD-L1-negative group’s survival was significantly improved against the comparator docetaxel.
In an open-label, phase 3 study (CHECKMATE-026) of patients with untreated stage III or recurrent NSCLC, nivolumab was compared to platinum-based chemotherapy.5 Nivolumab was not associated with a significantly longer PFS and the OS was similar across both arms. In patients with >5% PD-L1 expression (n=423), median PFS was 4.2 months with nivolumab and 5.9 months with chemotherapy (HR, 1.15; 95% CI, 0.91-1.45; P= .25). The safety profile of nivolumab was more favorable with treatment-related AEs (grade 3/4) of 18% and 51% for nivolumab and chemotherapy, respectively. CheckMate-026 demonstrated the superiority of nivolumab versus chemotherapy in this setting in patients with a tumor proportion score ≥1%.
An open-label, phase II study, (CheckMate 568) evaluated the efficacy and safety of nivolumab plus low-dose ipilimumab as first-line treatment of advanced/metastatic NSCLC.6 ORR was 30% overall and 41% and 15% in patients with ≥1% and <1% tumor PD-L1 expression, respectively. ORR increased with higher TMB, plateauing at 10 or more mutations/megabase (mut/Mb).
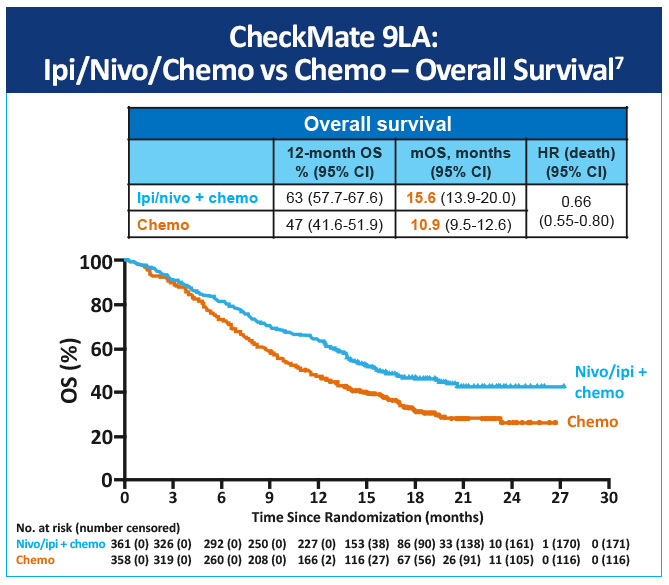
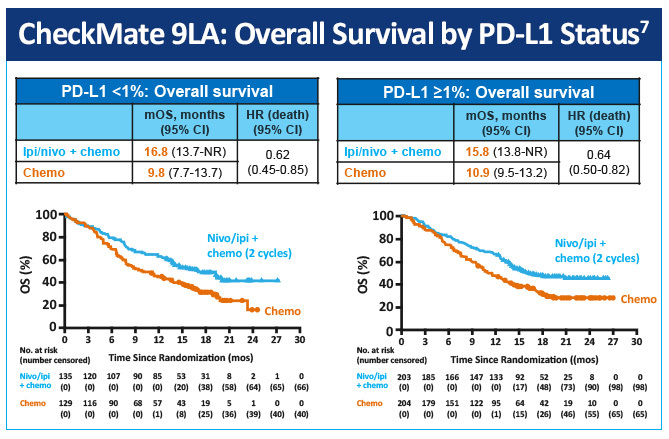
In a randomized, open-label, phase 3 trial (CheckMate-9LA) first-line nivolumab plus ipilimumab in combination with 2 lines of chemotherapy versus 4 cycles of chemotherapy alone in patients with advanced NSCLC was evaluated.7 At median follow-up (13.2 months [IQR 6.4-17.0]), median overall survival was 15.6 months (95% CI, 13.9-20.0) in the nivolumab plus ipilimumab and chemotherapy group versus 10.9 months (9.5-12.6) versus the monotherapy chemotherapy group (HR 0.66 [95% CI, 0.55-0.80]) (Figure 3). Nivolumab plus ipilimumab with two cycles of chemotherapy provided a significant improvement in overall survival by PD-L1 status versus chemotherapy alone (Figure 4). The most common grade 3-4 treatment-related adverse events were neutropenia (in 24 [7%] patients in the experimental group vs 32 [9%] in the control group), anemia (21 [6%] vs 50 [14%]), diarrhea (14 [4%] vs 2 [1%]), increased lipase (22 [6%] vs 3 [1%]), and asthenia (3 [1%] vs 8 [2%]). Serious treatment-related adverse events of any grade occurred in 106 (30%) patients in the experimental group and 62 (18%) in the control group.
Cemiplimab
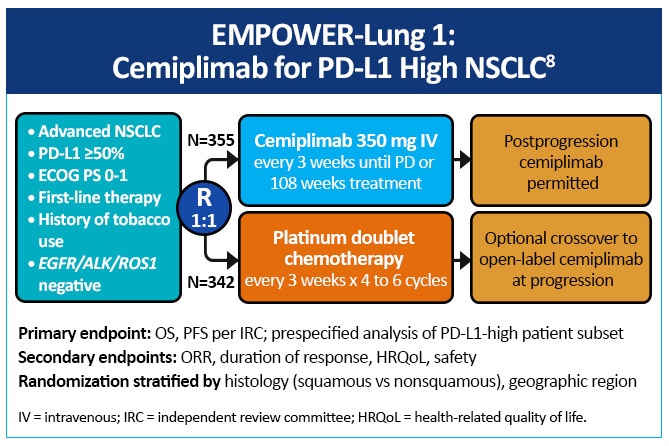
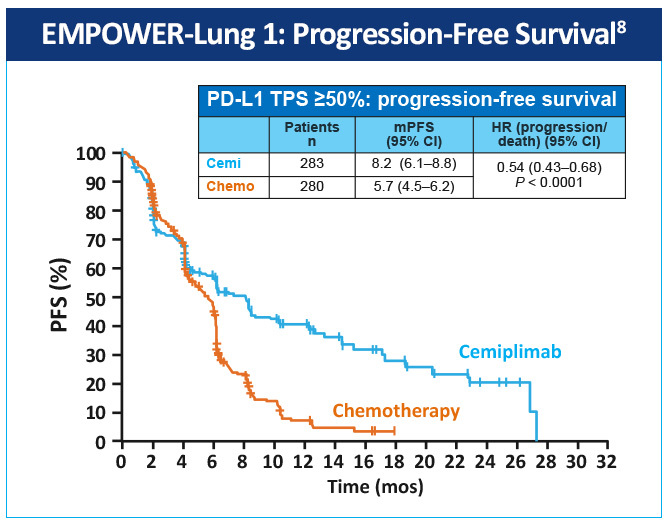
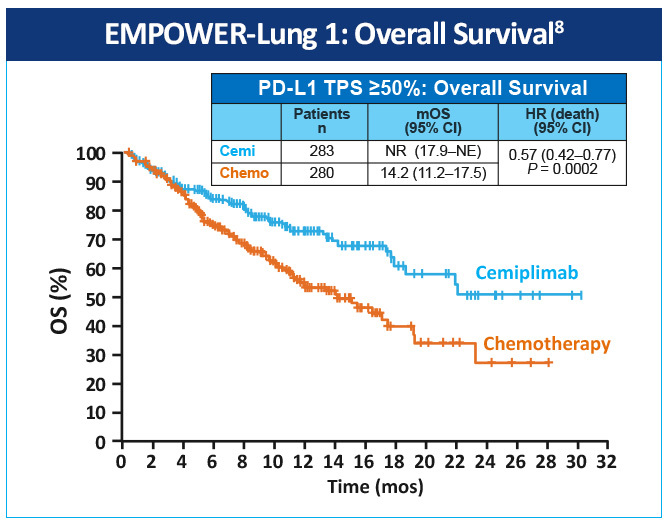
EMPOWER-Lung 1 was a multicenter, open-label phase 3 study evaluating cemiplimab, a PD-1 inhibitor, in the first-line treatment of advanced NSCLC with PD-L1 expression ≥50% based on the 22C3 assay (Figure 5).8 Patients treated with cemiplimab monotherapy versus chemotherapy exhibited improved progression-free survival 8.2 months (95% CI, 6.1-8.8) versus 5.7 months (95% CI, 4.5-6.2), for a 48% reduction in the risk of progression (HR 0.54 [0.43-0.68]; P< .0001) with cemiplimab (Figure 6). Median overall survival was 22.1 months (95% CI, 17.7-NE) with cemiplimab versus 14.3 months (95% CI, 11.7-19.2) with chemotherapy, for a 32% reduction in the risk of death (HR 0.68; 95% CI, 0.53-0.87, P= .0022; Figure 7). Moreover, serious (grade 3 or 4) adverse events were less frequent in patients receiving cemiplimab versus chemotherapy (28% vs 39%). These results led to the 2021 FDA and EMA approvals of cemiplimab for the first-line management of advanced NSCLC (including locally advanced who are not candidates for surgical resection or definitive chemoradiation) whose tumors have a tumor proportion score ≥50% and lack EGFR, ALK or ROS1 aberrations.
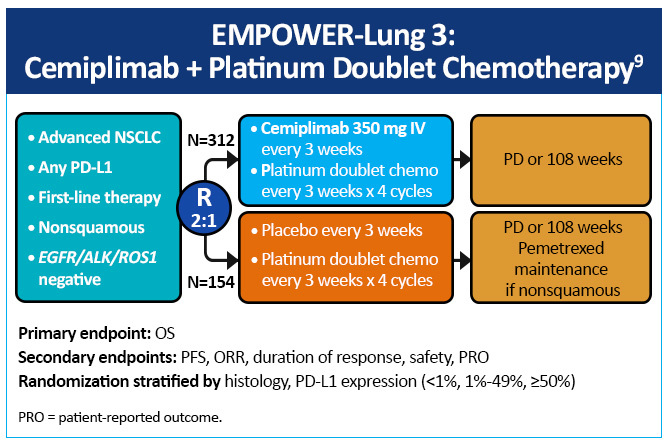
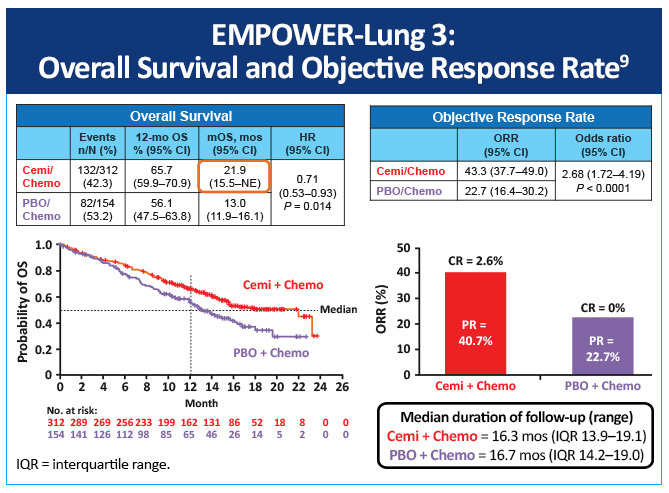
EMPOWER-Lung 3, a double-blind, placebo-controlled, phase 3 study examined cemiplimab plus platinum-doublet chemotherapy as first-line treatment of patients with NSCLC, irrespective of PD-L1 expression or histology (Figure 8).9 Impressively, this study was stopped early due to an overall survival benefit of 22 months with cemiplimab versus 13 months with chemotherapy (Figure 9). Cemiplimab showed efficacy in advanced NSCLC as both monotherapy and in combination with chemotherapy for both squamous and non-squamous histologies. Unlike prior studies, such as KEYNOTE-042 and CheckMate-227, which did not conclusively show a survival benefit in patients with PD-L1 expression <50%, these results for the first time show an unequivocal treatment benefit across all patients with NSCLC regardless of PD-L1 expression.
References
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. https://doi.org/10.1056/NEJMoa1606774
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomized, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. https://doi.org/10.1016/S1470-2045(16)30498-3
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. https://doi.org/10.1016/S0140-6736(18)32409-7
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. https://doi.org/10.1056/NEJMoa1507643
- Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. https://doi.org/10.1056/NEJMoa1613493
- Ready N, Hellmann MD, Awad MM, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): Outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992-1000. https://doi.org/10.1200/JCO.18.01042
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. https://doi.org/10.1016/S1470-2045(20)30641-0
- Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592-604. https://doi.org/10.1016/S0140-6736(21)00228-2
- Gogishvili M, Melkadze T, Makharadze T, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: A randomized, controlled, double-blind phase 3 trial [published online ahead of print, 2022 Aug 25]. Nat Med. 2022;10.1038/s41591-022-01977-y. https://doi.org/10.1038/s41591-022-01977-y