Biomarkers for selection of therapies for the first-line management of NSCLC
The need for predictive biomarkers is crucial for the effective management of NSCLC. For example, nivolumab is highly active in a very select group of patients.1 The use of archival tumor samples from pretreated patients in the pivotal phase 1 study may explain the differences in ORR according to PD-L1 expression. A phase 1 study demonstrated that anti-PD-1 antibody produced objective responses in approximately one in four to one in five patients with non-small-cell lung cancer, melanoma, or renal-cell cancer; the adverse-event profile does not appear to preclude its use.2 Preliminary data suggest a relationship between PD-L1 expression on tumor cells and objective response.
It is recognized that PD-L1 expression varies with the tumor microenvironment and thus PD-L1 expression at a single time point may not represent a dynamic immune response. Thus PD-L1 expression as a predictive biomarker may be difficult to implement. Further studies are needed to characterize immunologic correlates of response in the pretreatment and on-treatment setting. Research is ongoing to identify patients who are likely to respond to PD-1/PD-L1-inhibitors.
In the front-line setting, the pivotal KEYNOTE-024 trial revealed that pembrolizumab was better than chemotherapy in patients with advanced NSCLC and high PD-L1 expression, defined as a tumor proportion score (TPS) greater than 50%. In this patient subset, pembrolizumab significantly prolonged PFS and OS.3 The KEYNOTE-042 study found that pembrolizumab was superior to chemotherapy in patients with PD-L1 TPS >1%, although the OS benefits were greater in patients with higher PD-L1 expression (TPS >50%).4 In the KEYNOTE-189 trial, the combination of pembrolizumab plus chemotherapy in patients with advanced non-squamous NSCLC and no EGFR/ALK alterations significantly improved PFS and OS compared to chemotherapy alone. The largest benefit was observed in patients with PD-L1 TPS >50% (HR, 0.42), although patients with PD-L1 expression <1% also saw improvement (HR, 0.59).5 This data suggests that PD-L1 expression alone is not sufficient to predict which patient group will most benefit from immunotherapy.
Subsequently, tumor proportion score (TPS) levels for PD-L1 has emerged as the logical biomarker on which to guide molecular selection for NSCLC receiving PD-1/PD-L1 inhibitors. However, a number of issues related to the standardization of PD-L1 testing have been previously raised.6 This includes the standardization of the laboratory method of PD-L1 testing as well as determination of the appropriate specimen with which to measure PD-L1. In a recently reported study, the concordance between surgical specimens and corresponding biopsy specimens in terms of PD-L1 expression has been evaluated.7 In this study, PD-L1 expression was frequently discordant between surgical resection and matched biopsy specimens (the overall discordance rate =48%). Notably, the biopsy specimens underscored the PD-L1 status compared to the surgical specimens in almost all cases. These findings have an important relevance to the standardization of the procedure of PD-L1 measurement. Another concern with the use of PD-L1 testing is the dynamic status of this marker and changes in the PD-L1 level have been observed across the clinical course of NSCLC treatment.8 Whether a re-biopsy has to be made at each time of instituting PD-1/PD-L1 targeted treatment is not yet known. The appropriate cutoff value with which to consider a tumor specimen as PD-L1 positive has been variable among different clinical studies; with some studies using 1%, 5%, 10% or 25% as cutoff values.9,10 A recent analysis indicates that a benefit exists even for PD-L >1% tumors compared to PD-L1 <1% tumors, suggesting that a cutoff value of 1% is a more suitable value to use in clinical studies.11
Another potential biomarker for immune checkpoint inhibition is high tumor mutational burden (TMB). Tumor mutation burden is a quantitative biomarker that reflects the total number of mutations carried by tumor cells.12 Tumor cells with high TMB have higher levels of neoantigens, which are thought to help the immune system recognize tumors and incite an increase in cancer-fighting T cells and an anti-tumor response.13 However, some patients with both high and low mutational burdens failed to respond to therapy, suggesting that other factors are involved in immunotherapy responses. These findings suggest that the degree of intratumor heterogeneity may be associated with a differential response to checkpoint blockade, and that multiple factors are involved in tumor immune responses.
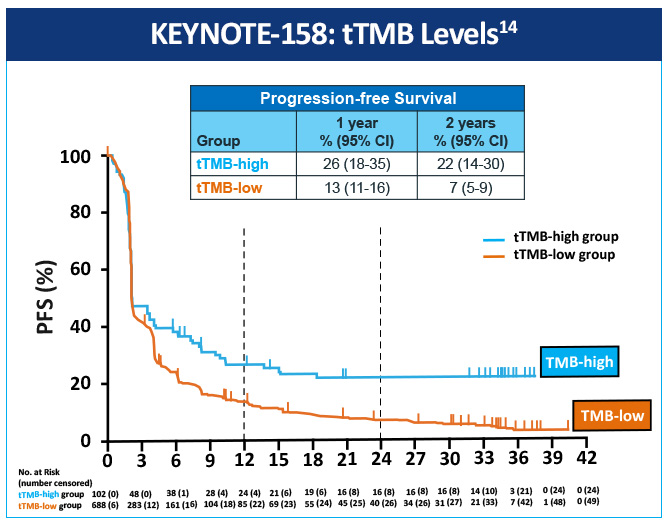
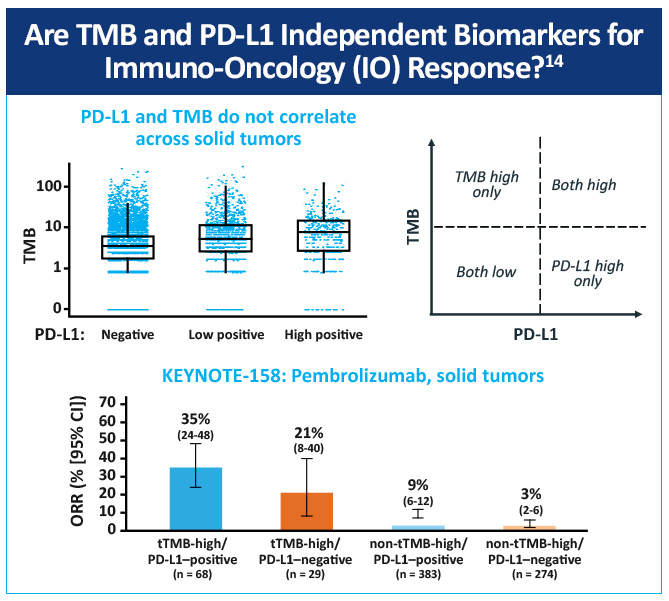
An open-label, non-randomized, phase 2 (KEYNOTE-158) study, assessed the association of high tissue TMB (tTMB-high) with outcomes in patients treated with pembrolizumab with selected, previously treated, advanced solid tumors.14 Objective responses were observed in 30 (29%; 95% CI, 21%-39%) of 102 patients in the tTMB-high group and 43 (6%; 95% CI, 5%-8%) of 688 in the non-tTMB-high group (Figure 1). The data suggest that tTMB-high status identifies a subgroup of patients who could have a robust tumor response to pembrolizumab monotherapy (Figure 2). tTMB could be a novel and useful predictive biomarker for response to pembrolizumab monotherapy in patients with previously treated recurrent or metastatic advanced solid tumors.
One study explored how mutational burden affects sensitivity to PD-1 blockade revealed an association between a higher nonsynonymous mutation burden and improved objective response, durable clinical benefit, and PFS.15 Another study investigated whether melanoma and NSCLC sensitivity to PD-1 and CTLA-4 treatment was enhanced in tumors where tumors had a high clonal neoantigen burden. Results showed that a combination of neoantigen clonality and neoantigen burden allowed for better discrimination of responders and non-responders than either metric alone.16 A recent study calculated TMB scores by whole exome sequencing in a subset of patients from a randomized phase 3 trial comparing nivolumab to platinum chemotherapy in treatment naive, advanced stage NSCLC (Checkmate 026).17 In patients with high TMB, PFS was improved (median PFS of 9.7 months vs 5.8 months (HR: 0.62 [95% CI, 0.38-1.00]); and the ORR was higher with nivolumab vs chemotherapy (46.8% v. 28.3%).
Other biomarker analyses presented at ASCO 2022 indicate which populations of patients may benefit most from frontline chemotherapy plus checkpoint inhibition. Neoadjuvant pembrolizumab combined with chemotherapy (NAPC) was associated with increased infiltration of CD8+ T cells, CD8+KLRG1+ effector cells, CD8+CD127+ memory cells, CD4+CD127+ memory cells, and CD20+ B cells, while neoadjuvant chemotherapy (NAC) was associated with increased infiltration of myeloid cells. Survival analysis showed that CD8+ T cells, CD8+KLRG1+ effector cells, and CD8+CD127+ memory cells were associated with favorable overall survival in both NAPC and NAC patients, while CD66b+ neutrophils was related to inferior overall survival.18 Still other studies have proposed use of whole-genome sequencing data to identify predictive features for response to immunotherapy drugs, with mutational burden being one predictive factor, along with specific single nucleotide variant mutations.19 Other mutations, such as the XIRP2 gene, are associated with improved progression-free survival following immune checkpoint inhibitor treatment. This suggests that the XIRP2 gene may also be a predictive factor for NSCLC patients who will respond positively to therapy.20
In addition to recognition of biomarkers associated with response to therapy in NSCLC, recognition of the potential immune-related and other adverse events unique to checkpoint inhibitors is important in optimizing therapy. Immune-related adverse events that may occur in some patients with NSCLC treated with checkpoint inhibitors include immune-mediated colitis, pneumonitis, and dermatologic adverse events. Recognition of these adverse events is crucial for oncologists who may be more familiar with the management of adverse events associated with chemotherapeutic agents. Evaluating potential adverse events is an important part of improving the safety of checkpoint inhibitor therapy in patients with NSCLC.21
References
- Sundar R, Cho BC, Brahmer JR, Soo RA. Nivolumab in NSCLC: Latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7(2):85-96. https://doi.org/10.1177/1758834014567470
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. https://doi.org/10.1056/NEJMoa1200690
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. https://doi.org/10.1056/NEJMoa1606774
- Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018:36(18 suppl):LBA4. https://doi.org/10.1200/JCO.2018.36.18_suppl.LBA4
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. https://doi.org/10.1056/NEJMoa1801005
- Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10(6):e0130142. https://doi.org/10.1371/journal.pone.0130142
- Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27(1):147-153. https://doi.org/10.1093/annonc/mdv489
- Gainor JF, Sequist LV, Shaw AT, et al. Clinical correlation and frequency of programmed death ligand-1 (PD-L1) expression in EGFR-mutant and ALK-rearranged non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(15 suppl):8012. https://doi.org/10.1200/jco.2015.33.15_suppl.8012
- Gettinger SN, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol. 2014;32(15 suppl):8024. https://doi.org/10.1200/jco.2014.32.15_suppl.8024
- Higgs BW, Robbins PB, Blake-Haskins JA, et al. 15LBA High tumoral IFNγ mRNA, PD-L1 protein, and combined IFNγ mRNA/PD-L1 protein expression associates with response to durvalumab (anti-PD-L1) monotherapy in NSCLC patients. Eur J Cancer. 2015;51(suppl 3):S717. https://doi.org/10.1016/S0959-8049(16)31937-2
- Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Crit Rev Oncol Hematol. 2016;101:75-85. https://doi.org/10.1016/j.critrevonc.2016.03.007
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29(suppl 4):iv192-iv237. Updated version published 15 September 2020 by the ESMO Guidelines Committee. https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer
- Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. 2017;15(1):133. https://doi.org/10.1186/s12916-017-0900-y
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet. 2020;21(10):1353-1365. https://doi.org/10.1016/S1470-2045(20)30445-9
- Rizvi NA, Brahmer JR, Ou SI, et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(15 suppl):8032. https://doi.org/10.1200/jco.2015.33.15_suppl.8032
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463-1469. https://doi.org/10.1126/science.aaf1490
- Peters S, Creelan B, Hellmann MD, et al. Abstract CT082: Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage IV or recurrent non-small cell lung cancer: An exploratory analysis of CheckMate 026. Cancer Res. 2017;77(13 suppl):CT082. https://doi.org/10.1158/1538-7445.AM2017-CT082
- Ren Y, Hui Z, Zheng L, et al. Shift of tumor-infiltrating immune cells after adding immune checkpoint inhibitors to neoadjuvant chemotherapy against NSCLC. J Clin Oncol. 2022;40(16 suppl):e21002. https://doi.org/10.1200/JCO.2022.40.16_suppl.e21002
- Pacewicz K, Kraszewski A, Medzin M, et al. Improved response prediction to immune checkpoint inhibition by combining TMB and WGS-based genomic features in NSCLC. J Clin Oncol. 2022;40(16 suppl):e21077. https://doi.org/10.1200/JCO.2022.40.16_suppl.e21077
- Chen H, Liu M, Luo Y, et al. XIRP2 mutation as an indicator stratified patients benefit from immune checkpoint inhibitors in NSCLC. J Clin Oncol. 2022;40(16 suppl):e14573. https://doi.org/10.1200/JCO.2022.40.16_suppl.e14573
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. V5.2022. September 26, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf