NSCLC Pathophysiology
Great strides have been made in delineating the molecular events that lead to non-small cell lung carcinoma (NSCLC), but a complete understanding of the pathogenesis continues to evolve. Historically, NSCLC has been considered a nonimmunogenic disease; however emerging evidence has demonstrated that the lack of an effective immune response is functional, rather than structural in nature.1
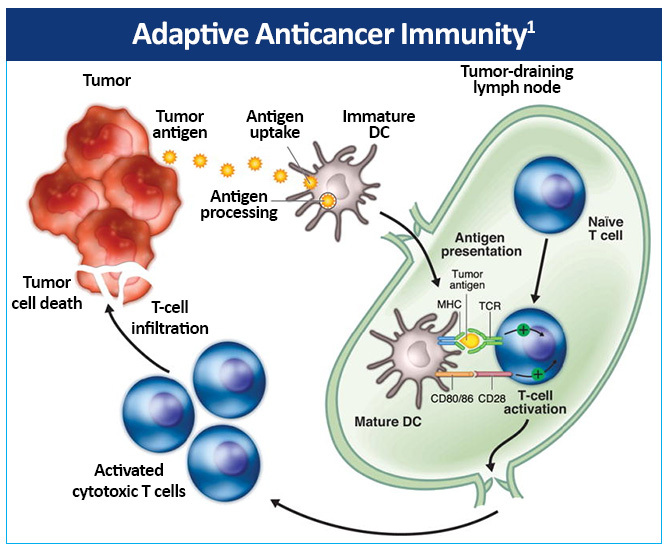
The adaptive anticancer immune response is initiated by immature dendritic cells (DCs), which are found in most tumors in humans and capable of capturing antigens released from cancer cells.1 Immature DCs subsequently undergo maturation and migrate to tumor-draining lymph nodes, presenting tumor antigens within major histocompatibility complex (MHC) molecules to naïve T cells (Figure 3). This triggers a protective T-cell response composed of specific CD4+ helper T (Th) cells and CD8+ cytotoxic T cells. T-cell activation requires interaction not only between the antigen-MHC complex on DCs and T-cell receptors but also among an array of co-stimulatory molecules, including CD80/86 on DCs and the CD28 receptor on T cells. The activated cytotoxic T cells infiltrate the tumor, thereby recognizing and killing tumor cells directly in an MHC-restricted fashion. Additionally, cytokines are secreted by the activated Th cells inducing inflammation and recruiting other immune cells to the tumor microenvironment to eliminate cancer cells. DCs also induce B-cell mediated antibody responses and nature killer (NK) cell activity.
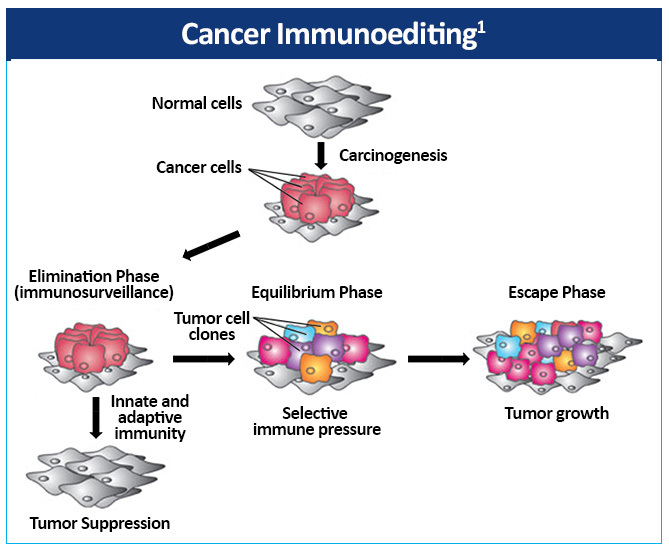
The complex relationship between the immune system and cancer can be
described by the hypothesis of cancer immunoediting, which encompasses the
dynamics of tumor development over time.2 The
immune system via immune surveillance can destroy transformed and/or malignant
cells.2,3 However, immune surveillance is not always successful,
resulting in ‘edited’ tumors that have escaped immune surveillance.
Cancer immunoediting is composed of three phases—elimination,
equilibrium, and escape (Figure 4).1 Both innate and adaptive
immunity belong to the elimination phase, also referred to as
immunosurveillance. If the immune system is unable to completely eradicate the
tumor cells, the microenvironment enters the next phase, equilibrium. This may
be a prolonged period of time during which tumor cells are maintained in a
state of functional dormancy by the immune system. However, a byproduct of this
selective pressure is the emergence and promotion of tumor cell variants with
decreased immunogenicity, thereby increasing resistance to immunologic attack.
Following this phase, tumor cell variants with low immunogenicity can enter the
escape phase, during which the immunologically sculpted tumor successfully
evades the immune system and grows sufficiently to be clinically detected.
In this favorable
immunoediting environment, tumors can progress where the antitumor adaptive and
innate response are blocked. Several immune protumor effector mechanisms are
upregulated by chronic inflammation, leading to the hypothesis that
inflammation promotes carcinogenesis and tumor growth by altering the balance
between protumor and antitumor immunity. In this scenario, the immune system
plays dual roles with host-protective and a tumor-promoting activities.
Immune checkpoints refer inhibitory pathways in the immune system that are crucial for maintaining normal self-tolerance and modulating the duration and amplitude of physiological immune responses in peripheral tissues, in order to minimize collateral tissue damage.4 Certain immune checkpoints can be exploited by tumors as immune resistance mechanisms.
Two of the most investigated checkpoint receptors in terms of immunotherapeutic targets for NSCLC are cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) receptor, which downregulate T-cell activation, proliferation, and function through different mechanisms.5,6
CTLA-4 is expressed on the surface of T cells after activation and competes with the co-stimulatory T-cell CD28 receptor for CD80/86 expressed by antigen-presenting cells (APCs), providing an inhibitory signal to the T cell (Figure 5).1
Large numbers of Treg cells that constitutively express high levels of CTLA-4 on their surface and directly inhibit T-cell proliferation have been found in NSCLC tumors.7,8
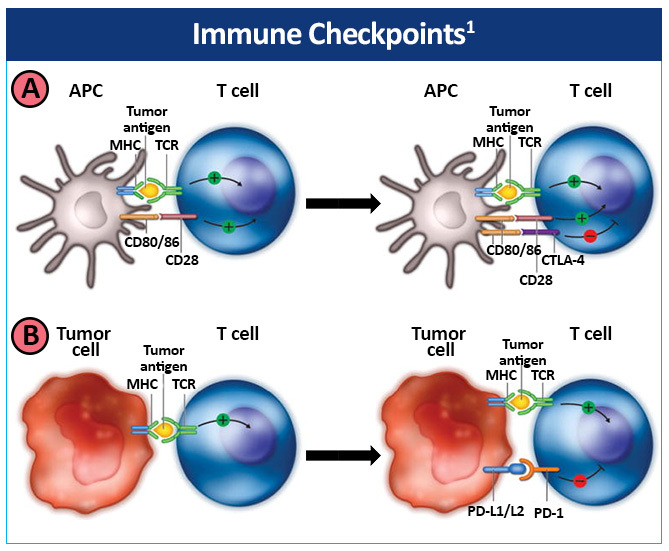
Tumors can develop immune resistance via the PD-1 pathway.1 Upregulation of the PD-1 receptor on activated T cells and subsequent binding to one of its ligands, programmed death ligand-1 (PD-L1) or PD-L2, provide an inhibitory signal during the effector phase of the T-cell response, reducing cytokine production, cell proliferation, and cell survival signaling. PD-1 is also expressed at high levels on Treg cells, enhancing their proliferation in the presence of a PD-1 ligand. In addition, PD-1 may be induced on activated NK cells, thereby limiting their lytic activity. PD-L1 and PD-L2 are also usually expressed on tumor cells. Tumor-infiltrating CD8+ T cells have shown increased PD-1 expression that was associated with impaired immune function in NSCLC.9 In addition, PD-L1 expression has also been found to be upregulated on NSCLC tumor cells.10
References
- Carbone DP, Gandara DR, Antonia SJ, Zielinski C, Paz-Ares L. Non-small-cell lung cancer: Role of the immune system and potential for immunotherapy. J Thorac Oncol. 2015;10(7):974-984. https://doi.org/10.1097/JTO.0000000000000551
- Ostrand-Rosenberg S. Immune surveillance: A balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11-18. https://doi.org/10.1016/j.gde.2007.12.007
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570. https://doi.org/10.1126/science.1203486
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer.
2012;12(4):252-264. https://doi.org/10.1038/nrc3239 - Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+) CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766-4772. https://pubmed.ncbi.nlm.nih.gov/11406550/
- Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168(9):4272-4276. https://doi.org/10.4049/jimmunol.168.9.4272
- Singh PP, Sharma P, Krishnan G, Lockhart AC. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol Rep (Oxf). 2015;3(4):289-297. https://doi.org/10.1093/gastro/gov053
- Chen DS, Irving BA, Hodi FS. Molecular pathways: Next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580-6587. https://doi.org/10.1158/1078-0432.CCR-12-1362
- Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7(5):389-395. https://doi.org/10.1038/cmi.2010.28
- Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: A 5-year-follow-up study. Tumori. 2012;98(6):751-755. https://doi.org/10.1177/030089161209800612