Targeting NSCLC based on appropriate application of clinical biomarkers
The treatment landscape of driver-negative NSCLC is rapidly evolving.1 Immune-checkpoint inhibitors, specifically those targeting PD-1 or PD-L1, have demonstrated durable efficacy in a subset of patients with NSCLC, becoming the cornerstone of first-line therapy. Research is underway to identify biomarkers that might predict which patients respond best to PD-1 pathway inhibitors, the most promising of which may be the level of PD-L1 expression in the tumor microenvironment.
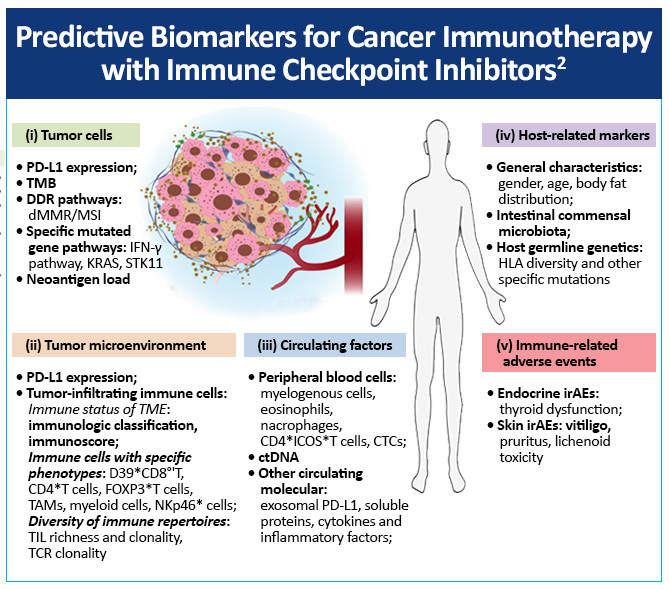
Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors include
- Tumor cells
- Tumor microenvironment
- Circulating factors
- Host-related markers
- Immune-related adverse events
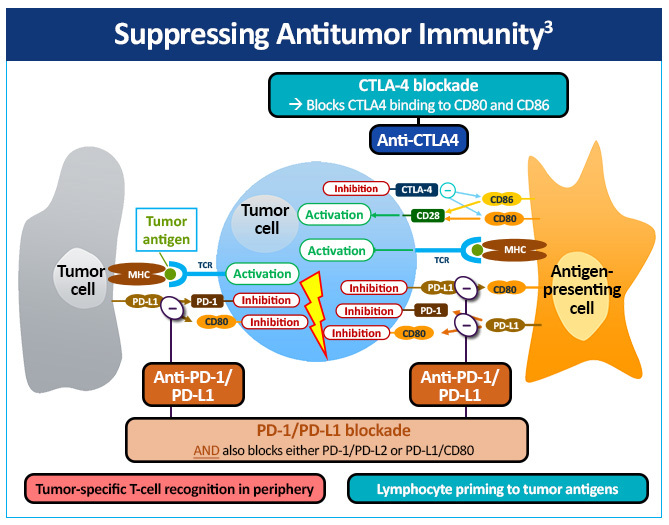
The immune checkpoint inhibitors that are currently most advanced in clinical development inhibit cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed cell death ligand 1 (PD-L1) (Figure 2).3
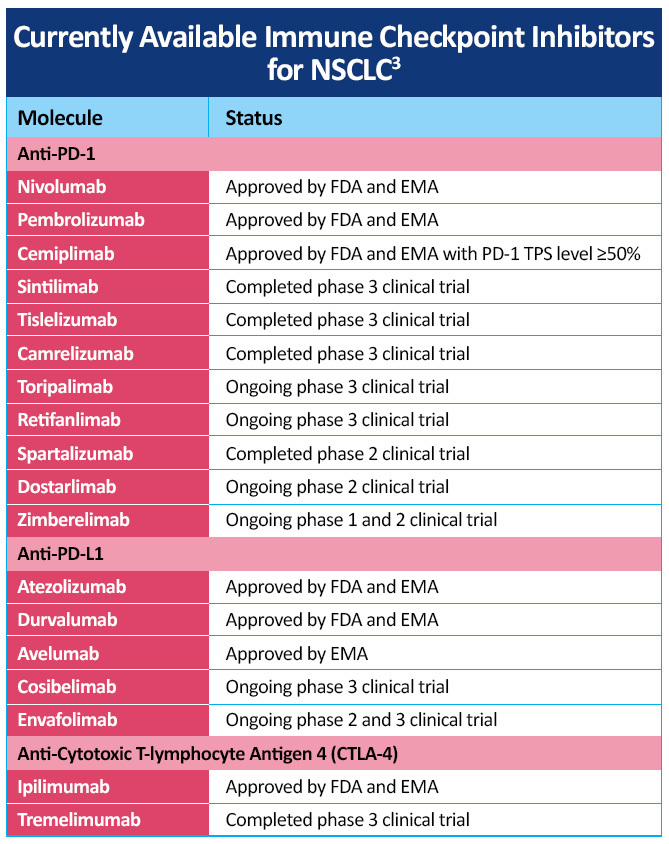
A number of checkpoint inhibitors currently have approved indications in the treatment of patients with NSCLC, including CTLA-4 inhibitor, PD-1 and PD-L1 inhibitors, both as monotherapy and in combination with chemotherapy (Table 2)3. Additional checkpoint inhibitors are being investigated in various stages of NSCLC with data from a number of studies are in progress and new advances are being made in the management of NSCLC, including developments in the later-generation immunotherapies cemiplimab, camrelizumab, sintilimab, and tislelizumab for NSCLC.
Additional therapies are in development targeting checkpoints other than PD-1 and PD-L1, such as LAG-3 and TIM-3. These proteins are expressed on immune cells and can interact with major histocompatibility complex class II molecules. LAG-3 can also downregulate T-cell cytokine production and promote regulatory T-cell expansion. In a study of patients with NSCLC, it was shown that those with elevated LAG-3 expression in T cells had shorter PFS when receiving PD-1-directed agents. TIM-3 is also expressed in tumor-infiltrating lymphocytes in several cancers, including NSCLC, and its activation can induce T-cell tolerance, opening the door for possible combinations of checkpoint inhibitors in certain patients.4
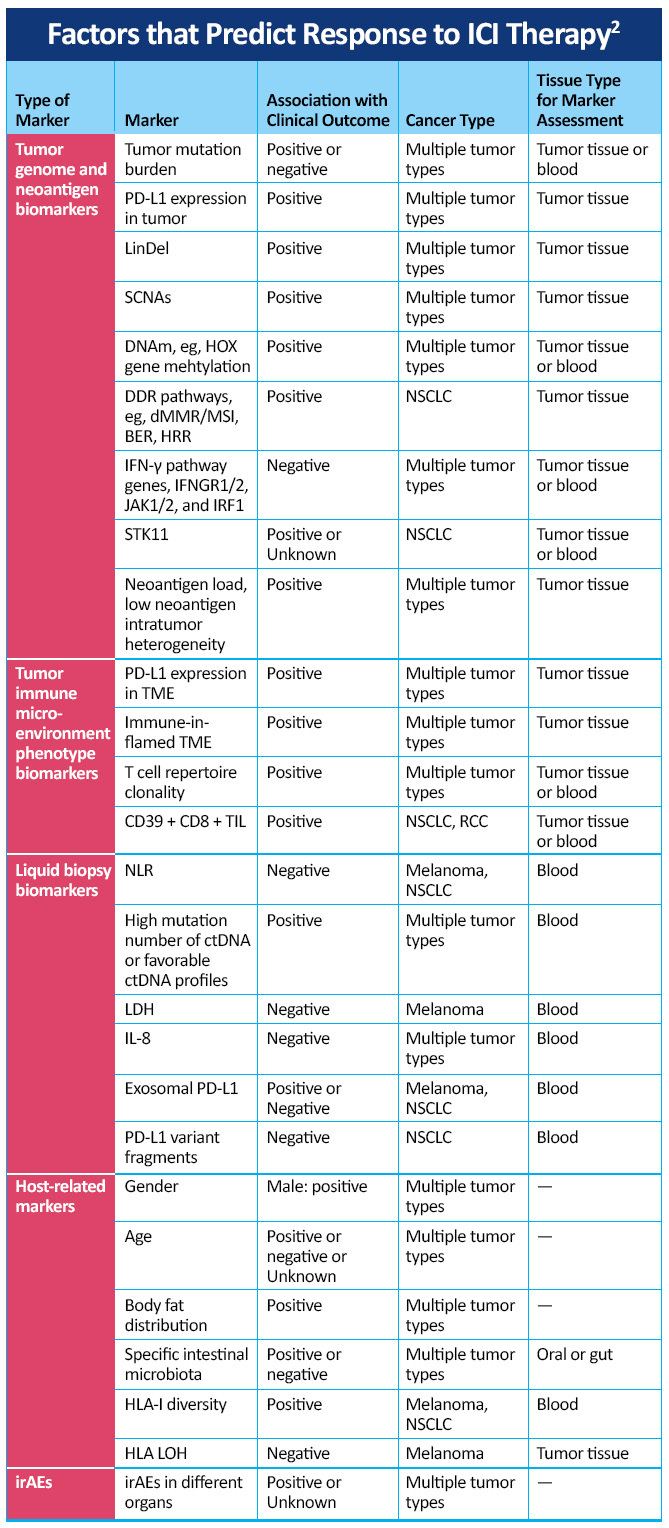
Interpretation of biomarker data remains an important priority for medical oncologists managing patients with advanced NSCLC. Current testing strategies include use of the tumor proportion score (TPS) for PD-L1 positivity and tumor mutational burden (TMB), as well as a variety of other testing modalities. Recognizing the importance of TPS of PD-L1 positivity and TMB is crucial in the selection of appropriate therapy. By recognizing these biomarkers and in an immersive program enabling visualization of the key mechanisms involved in checkpoint inhibition in lung cancer, oncologist and other health care professionals can understand the important mechanisms involved in checkpoint inhibition, key differences between PD-1 and PD-L1 inhibition, and clinical data showing how biomarkers and PD-L1 expression levels inform the use of treatments in patients with PD-L1 tumor proportion scores of 50% or higher and in the range of 1% to 49% in trials such as EMPOWER-Lung 1, EMPOWER-Lung 3, CheckMate-227, and KEYNOTE-042.
References
- Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18(10):625-644. https://doi.org/10.1038/s41571-021-00520-1
- Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. https://doi.org/10.1186/s40364-020-00209-0
- Suraya R, Tachihara M, Nagano T, Nishimura Y, Kobayashi K. Immunotherapy in advanced non-small cell lung cancers: Current status and updates. Cancer Manag Res. 2022;14:2079-2090. https://doi.org/10.2147/CMAR.S366738
- Sanborn RE, Schneiders FL, Senan S, Gadgeel SM. Beyond checkpoint inhibitors: Enhancing antitumor immune response in lung cancer. Am Soc Clin Oncol Educ Book. 2022;42:1-14. https://doi.org/10.1200/EDBK_350967