Clinical Trials for First-Line
Therapies in NSCLC
Preferred immunotherapeutic strategies for patients with treatment-naive, advanced or metastatic NSCLC include immune checkpoint inhibitors (ICI monotherapy or ICIs in combination with platinum-based chemotherapy).1 Notably, clinical decision-making is partially driven by the degree of PD-L1 expression. For patients with ≥50% PD-L1 expression, NCCN guidelines recommend single-agent atezolizumab, cemiplimab, or pembrolizumab (discussed in further detail below) or triplet therapy with cemiplimab or pembrolizumab with platinum doublet/histology-based chemotherapy.1 For those with PD-L1 expression between 1% and 49%, preferred options include triplet therapy with cemiplimab or pembrolizumab with platinum doublet/histology-based chemotherapy.1
First-Line Monotherapy for Metastatic NSCLC
Pembrolizumab
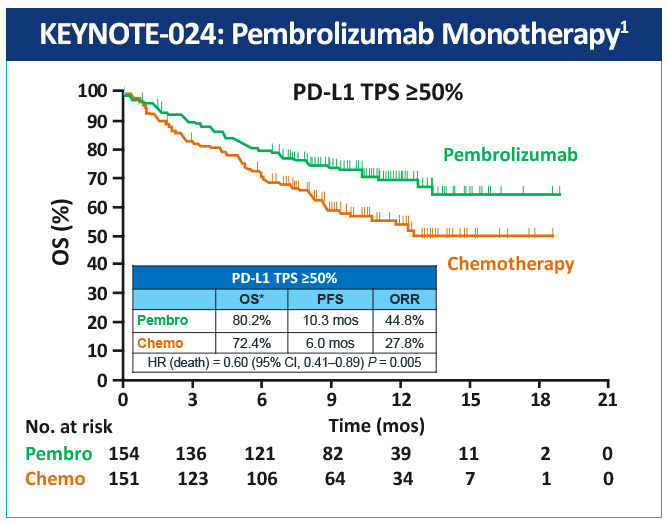
The KEYNOTE-024 study, a randomized, phase 3 study evaluated pembrolizumab as monotherapy compared with standard of care platinum-based chemotherapy in the treatment of patients with metastatic NSCLC whose tumors were marker-negative (EGFR or ALK) and expressed high levels of PD-L1 (≥50% of tumor cells).2 Median progression-free survival (PFS) was 10.3 months with pembrolizumab versus 6 months with chemotherapy; overall survival (OS) at 6 months was 80% vs 72% (HR=0.60); and 1-year OS was 70% vs 54%, respectively (Figure 1). Toxicity was lower with pembrolizumab than with chemotherapy (grade 3/4 adverse events (AEs): 27% vs 53%, respectively), and the incidence of all AEs was lower with pembrolizumab. Based on the results of this study, the FDA and EMA approved pembrolizumab for first-line treatment of patients with metastatic NSCLC whose tumors have high PD-L1 expression (TPS >50%) with no EGFR or ALK genomic tumor aberrations.
In KEYNOTE-042, a randomized, open-label, phase 3 study, first-line pembrolizumab monotherapy in patients with locally advanced or metastatic NSCLC and low PD-L1 TPS was evaluated compared with platinum-based chemotherapy of choice.3 Overall survival was significantly longer in the pembrolizumab arm than in the chemotherapy group in all three TPS populations (≥50%, HR=0.69, P = .0003; ≥20%, HR=0.77, P = .0020, and ≥1%, HR=0.81; P = .0018) (Figure 2). The median survival values by TPS population were 20.0 months for pembrolizumab versus 12.2 months for chemotherapy, 17.7 months versus 13.0 months, and 16.7 months versus 12.1 months, respectively.

Cemiplimab
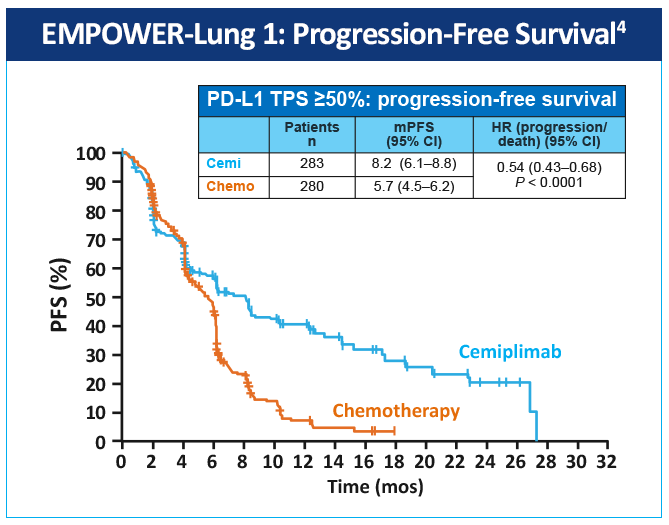
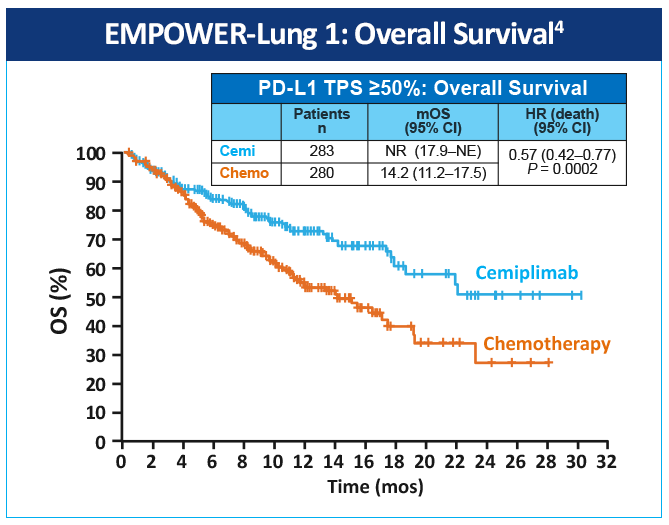
EMPOWER-Lung 1 was a multicenter, open-label phase 3 study evaluating cemiplimab, a PD-1 inhibitor, in the first-line treatment of advanced NSCLC with PD-L1 expression ≥50% based on the 22C3 assay (Figure 3).4 Patients treated with cemiplimab monotherapy versus chemotherapy exhibited improved PFS of 8.2 months (95% CI, 6.1-8.8) versus 5.7 months (95% CI, 4.5-6.2), for a 48% reduction in the risk of progression (HR=0.54 [0.43-0.68]; P < .0001) with cemiplimab (Figure 4). Median OS was 22.1 months with cemiplimab versus 14.3 months with chemotherapy, for a 32% reduction in the risk of death (HR=0.68; 95% CI, 0.53-0.87, P = .0022; Figure 5). Moreover, serious (grade 3 or 4) AEs were less frequent in patients receiving cemiplimab versus chemotherapy (28% vs 39%). These results led to the 2021 FDA and EMA approvals of cemiplimab for the first-line management of advanced NSCLC (including locally advanced who are not candidates for surgical resection or definitive chemoradiation) whose tumors have a tumor proportion score ≥50% and lack EGFR, ALK or ROS1 aberrations.
EMPOWER-Lung 3, a double-blind, placebo-controlled, phase 3 study examined cemiplimab plus platinum-doublet chemotherapy as first-line treatment of patients with NSCLC, irrespective of PD-L1 expression or histology (Figure 6).5 Impressively, this study was stopped early due to an OS benefit of 22 months with cemiplimab versus 13 months with chemotherapy (Figure 7). Cemiplimab showed efficacy in advanced NSCLC as both monotherapy and in combination with chemotherapy for both squamous and non-squamous histologies. Unlike prior studies, such as KEYNOTE-042 and CheckMate-227, which did not conclusively show a survival benefit in patients with PD-L1 expression <50%, these results for the first time show an unequivocal treatment benefit across all patients with NSCLC regardless of PD-L1 expression.
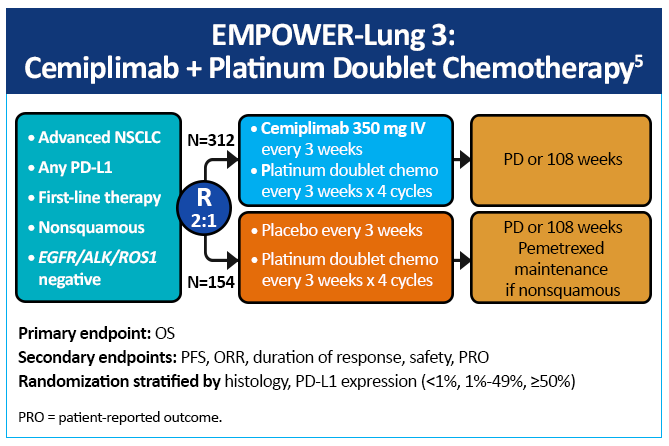

Notably, five-year data from EMPOWER-3 presented at the International Association for the Study of Lung Cancer (IASLC) meeting in September 2025 showed continued durable long-term benefits with the use of cemiplimab + chemotherapy versus chemotherapy alone, with safety profiles that remained consistent with earlier published data.6
Atezolizumab
In the IMpower 110 study – a randomized, open-label, phase 3 trial involving patients with metastatic nonsquamous or squamous NSCLC who had not previously received chemotherapy and who had PD-L1 expression on at least 1% of tumor cells or at least 1% of tumor-infiltrating immune cells – patients were assigned to receive atezolizumab or chemotherapy.7 Atezolizumab treatment resulted in significantly longer overall survival than platinum-based chemotherapy among patients with NSCLC with high PD-L1 expression, regardless of histologic type.7 (Figure 8)
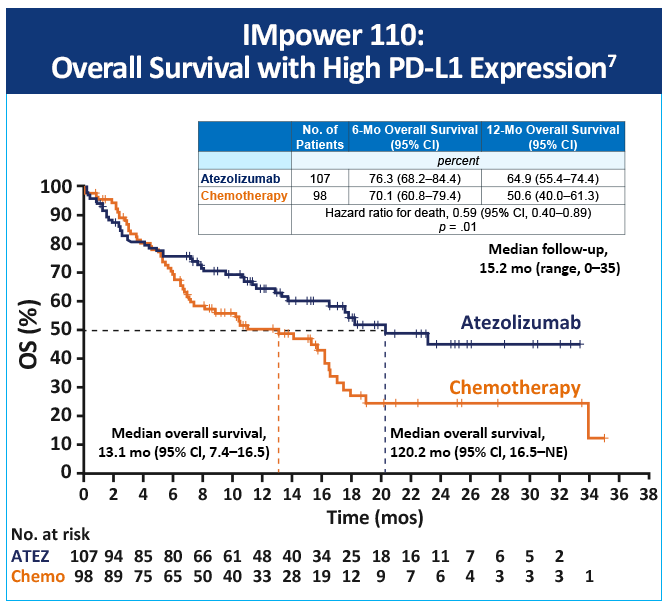
An updated overall survival analysis from IMpower110 found that statistical significance for OS was not revealed in the high-or-intermediate expression wild-type (WT) group, and, as a result, OS in the any PD-L1 expression WT group was not formally tested.8 This update supported using atezolizumab in treatment-naïve, metastatic WT NSCLC with high PD-L1 expression.
References
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Version 8.2025. August 15, 2025. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375:1823-1833. https://doi.org/10.1056/NEJMoa1606774
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819-1830. https://doi.org/10.1016/S0140-6736(18)32409-7
- Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592-604. https://doi.org/10.1016/S0140-6736(21)00228-2
- Gogishvili M, Melkadze T, Makharadze T, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: A randomized, controlled, double-blind phase 3 trial. Nat Med. 2022;28:2374-2380. https://doi.org/10.1038/s41591-022-01977-y
- Baramidze A, Makharadze T, Gogishvilli M, et al. Cemiplimab plus chemotherapy vs chemotherapy in advanced NSCLC: 5-year results from phase3 EMPOWER-Lung 3 part 2 trial. IASLC Annual Meeting. September 6-9, 2025. Abstract MA10.09. https://cattendee.abstractsonline.com/meeting/21151/Session/210
- Herbst R, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328-1339. https://doi.org/10.1056/NEJMoa1917346
- Jassem J, de Marinis F, Giaccone G, et al. Updated overall survival analysis from IMpower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. 2021;16:1872-1882. https://doi.org/10.1016/j.jtho.2021.06.019